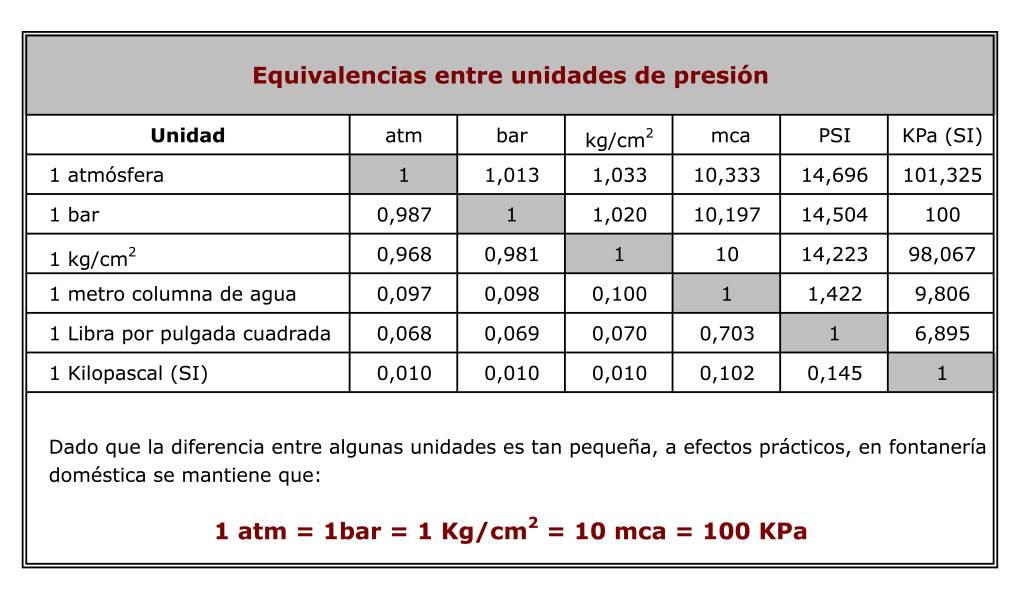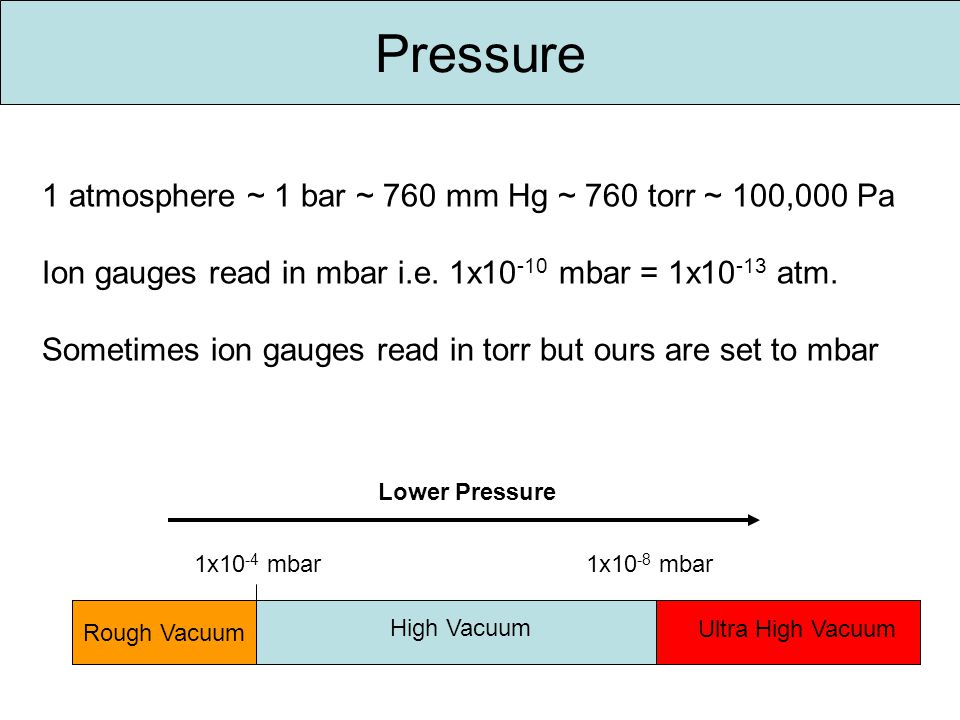
Pressure 1 atmosphere ~ 1 bar ~ 760 mm Hg ~ 760 torr ~ 100,000 Pa Ion gauges read in mbar i.e. 1x mbar = 1x atm. Sometimes ion gauges read. - ppt download

How to convert Atmospheric pressure to Pascal (atm-Pa) and Pascal to Atmospheric pressure (Pa-atm). - YouTube

pressure, conversion units into defferent units,atm,bar,torr,psi,Pascal,mmHg, numerical,and examples - YouTube

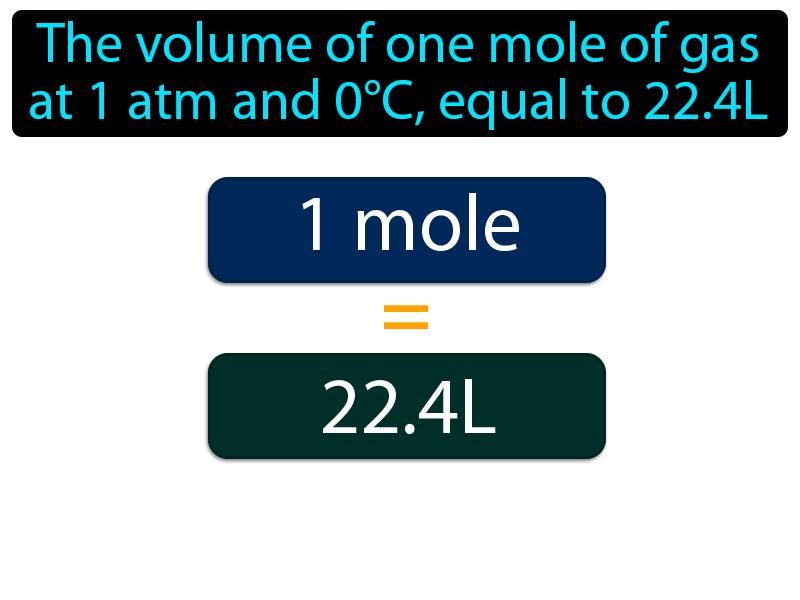
Is this incorrect? Standard Condition = 298K, 1 ATM and 1 M, STP = 273K, 1 ATM, 22.4 L. Shouldn't the card say Standard Condition is 298K? : r/Mcat

What is the molecular weight of a gas that has a density of 2 g/L at a temperature of 300 K and a pressure of 2 atm ?